There is always a debate among Electrical Engineers whether stationary battery rooms are classified as hazardous area (explosive) or non-hazardous area (non-explosive). The argument is always the phenomenon that batteries emit hydrogen during charging process. Knowing that hydrogen is a combustible gas, thus may cause ignition inside the battery room.
Lower Explosive Limit
To understand whether the hydrogen concentration emitted from the charging of the batteries will cause explosion or not is to first understand what makes an explosive atmosphere. What is the lower explosive limit for hydrogen?
Lower Explosive Limit (LEL), is defined as the lowest concentration (by percentage) of a gas or vapor in air that is capable of producing a flash of fire in presence of an ignition source (arc, flame, heat). For hydrogen, the gas emitted by the batteries during charging has a lower explosive limit of 4% by volume.
Sample Calculation
Assuming a room containing the batteries has a dimension 4m x 5m x 4m (W x L x H) or 80 cubic meters in volume containing a battery with C10 rating 2000 Ah. The battery voltage is 120V with a single string of 2 volts/cell or 60 cell battery.
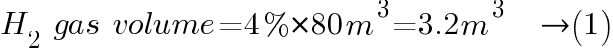
From Ventilation Requirements for Stationary Battery Rooms Table 1, the typical charging current for lead acid battery is 1 mA/Ah (float charge) and 4 mA/Ah (boost charge).
How much hydrogen gas will the batteries produce to reach the 4% lower explosive limit?
At float charge:
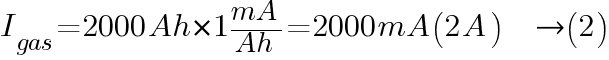
At boost charge:
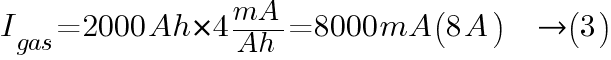
Substituing the values in equation (2) from Ventilation Requirements for Stationary Battery Rooms.
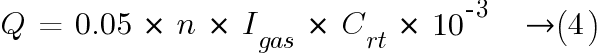
At float charge:
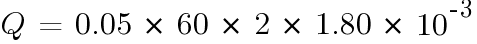
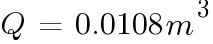
The number of hours of for the batteries to emit 4% of the room volume will be:
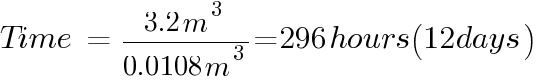
At boost charge:
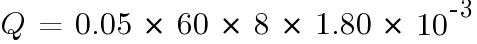
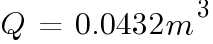
The number of hours of for the batteries to emit 4% of the room volume will be:
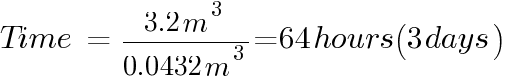
Please note that boost charge will only happen for a few hours and not usually recommended as it will shorten the life of the battery.
Required Air Change
To prevent the formation on an explosive atmosphere inside the battery room, the air change required in our example above is only once every 3 days for boost charge or 12 days for float charge condition.
Even a normal entry-exit of personnel inside the room will disperse or dilute the emitted hydrogen gas by the battery during charging thus no explosive atmosphere formation will occur. It is still recommended though to consult a competent HVAC practitioner in this case.
